Alcohol and Gaming Commission of Ontario (AGCO) Inspectors continue to find and seize non-compliant veterinary products in stables, including First Choice Equine (FCE). Products that do not meet the requirements for labelling for veterinary use under the Food and Drugs Regulations, including regulation C.R.C. c 870 , under The Food and Drugs Act (Canada) are prohibited pursuant to the Rules of Racing in Ontario.
Using products that have not been approved by Health Canada increases the risk that they were not evaluated and monitored for safety, quality, effectiveness and in accordance with Health Canada Standards. Labels give key information about the safe and proper use of veterinary drugs, and they identify the product, its ingredients, and directions for proper administration.
The use of products without proper labels is a violation of SB R. 6.46.01 and TB R. 15.31.01, which prohibit the use of a drug, substance or medication for a horse “which has not been labeled for veterinary use under the Food and Drugs Regulations under The Food and Drugs Act (Canada) or, (…) has not been prescribed by a veterinarian after conducting an examination of the horse and determining that the drug, substance or medication is medically required by the horse and the drug, substance or medication is used only for that horse in accordance with the prescription issued by the veterinarian.”
As such, the use of these products, including certain First Choice Equine (FCE) products, will be treated as violations of the Rules of Racing because they do not comply with the applicable labelling laws, regulations, rules and/or requirements.
It is your responsibility to ensure that any products you administer to racehorses comply with The Food and Drugs Act (Canada), its regulations and the Rules of Racing
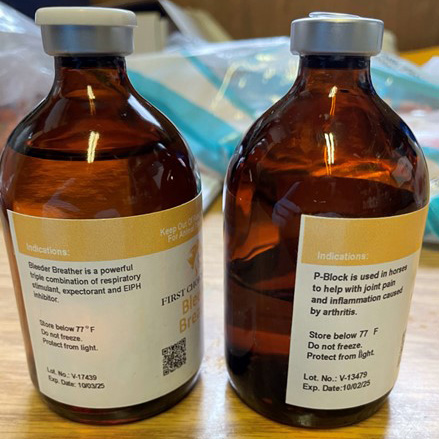
Please see below for some examples of what to look for when reviewing product labels as well as examples of improper product labelling.
For more information, please contact AGCO Veterinary Services at 1-855-261-6821 or vetclerks@agco.ca.
Examples of Canadian labelling requirements
Principal Display Panel of inner label must contain ALL of the following:
- Brand Name
- If no brand name exists, proper/ common name is acceptable
- Dosage Form
- Notification Number
- NOT REQUIRED, highly recommended
- “Veterinary Health Product” statement in both English and French
Any Panel of inner and outer labels must contain:
- Name and Address of Canadian manufacturer, importer, or representative
- Adequate Directions for Use
- Recommended Use or Purpose
- Intended Species
- Directions for Use
- Recommended Dose
- Duration of Use
- Route of Administration
- Important Safety Information
- Warnings and/or Cautions (if applicable)
- Recommended Storage Conditions (if applicable)
- French Translation
- Ingredients
- Proper or Common Names of Active Ingredients
- Quantity of Active Ingredient(s) per Dosage Unit
- List of Excipient (non-active) Ingredients
- Lot Number
- Expiry Date or Shelf Life
- Net Amount of total health product
For drug product containers that are too small to carry all the labelling requirements
NOTE: must be dispensed with an outer label containing all labelling requirements
Inner Label must contain:
- Common or Proper Name
- Brand Name is sufficient for drug products with more than one medicinal ingredient
- Quantitative Amounts or potency of the drug’s medicinal ingredient (e.g. mg/mL)
- Not required if the drug product contains more than on medicinal ingredient
- Drug Identification Number (DIN) for veterinary drug products
- Route of Administration
- Not required for oral administrations
- Name of the Manufacturer
- Lot Number
- Expiration Date
- Net Amount of total drug product
SOURCE: Health Canada, Guidance on veterinary drug labelling: Labelling unique types of packages
Some common examples of labels missing some required information:
Consistently absent on inner label:
✘ Proper/ common name immediately following brand name
✘ Dosage form of the product
✘ Standard of manufacture
✘ Use of the term “sterile”
✘ Drug Identification Number (DIN)
✘ Use of the statement “Veterinary Use Only”
- First Choice Equine Products contain the phrase “Administer on the advice of a veterinarian”
- C.01.600 of the Food and Drug Regulations explicitly states the phrase “Veterinary Use Only” must be used in both English and French
✘ Canadian Name and Address Information
✘ French translation of Adequate Directions for Use
Notably absent from the inner label of most drug products:
✘ Route of administration as per adequate directions for use
✘ Instruction to “consult package insert” or outer carton for adequate directions for use
- Implies the product was not dispensed with an outer carton or package insert
- Not required for Pentosan since it meets the minimum requirements
In the event that the veterinary drug products are dispensed with outer labels containing the necessary labelling requirements and the immediate container can be considered “small packaging”:
Consistently or notably absent on inner label:
✘ Drug Identification Number (DIN)
✘ Route of Administration